
In a western immunoblot, the primary antibody could be ‘monoclonal’ or ‘polyclonal’, and the choice made by the user is typically based on the most effective antibody available for the given protein and/or the specific information needed. For the detection of your GFP fusion proteins, we are using an antibody that binds specifically to GFP, and should therefore detect each fusion protein regardless of what the ‘fusion partner’ is. The image can be ‘reversed’ for clarity, and these images typically are, prior to presentation in a report or publication.Īntibodies as tools for detection of proteins:Īmong all the proteins extracted from the transfected tumor cells and resolved in the electrophoresis step, the western blot allows the detection of a single protein, or more specifically, a single protein domain or epitope, on the blot.
Ultimately, this produces an image of your blot with ‘white’ glowing bands on a black background. The chemiluminescent substrate mixture for HRP consists of peroxide buffers and a proprietary variation of luminol designed to generate light from very small quantities of protein. This is called chemiluminescent detection (using chemical reactions to generate light) and the emitted photons are captured using the CCD camera of the BioChemi imaging unit.

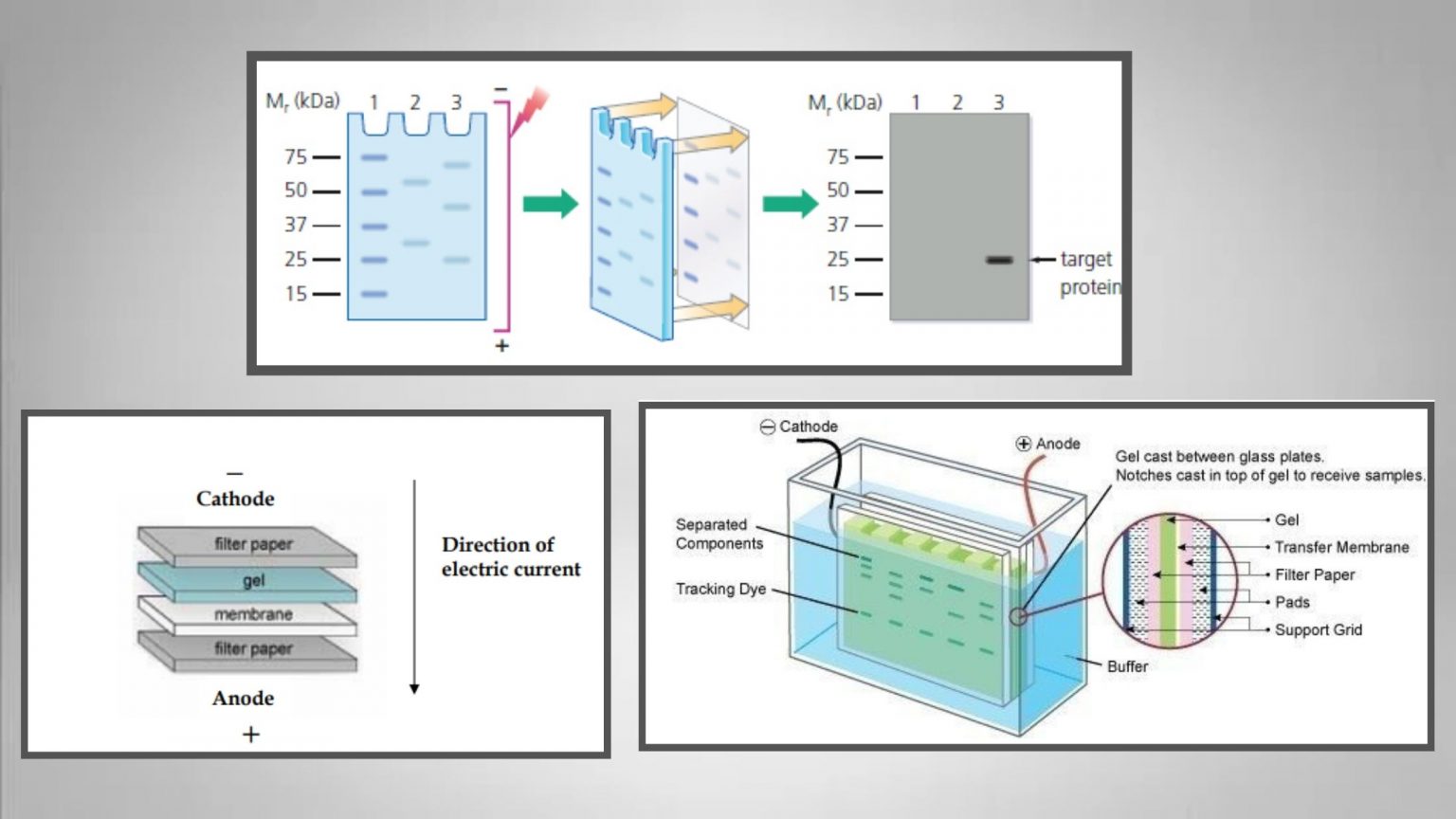
anti-mouse antibodies or anti-rabbit antibodies) is covalently linked to an enzyme (typically horseradish peroxidase, HRP) that catalyzes a localized light-generating reaction in the presence of substrate. This secondary antibody, which is raised against antibodies of a specific species, (e.g. The excess primary antibody is removed after a brief incubation period, then the blot is “washed” with buffer to remove non-specifically or weakly bound antibodies.Īfter this, a secondary antibody that recognizes the first (primary) antibody is incubated on the blot. For our purposes this target protein is GFP, the common “C-terminal tag” of all the fusion proteins. After preliminary incubation of the membrane in excess ‘inert’ protein (eg, milk) to block non-specific protein binding reactions, the membrane is then incubated with a primary antibody, which is an antibody that was generated to specifically recognize the ‘protein of interest’. To perform an immunoblot, the separated proteins within the gel are first transferred onto a membrane (usually nitrocellulose or PVDF) that can be treated with antibody solutions. Overview of a typical immunoblot procedure:Ī specific, single protein on a gel can be visualized through immunoblot analysis with an antibody that recognizes the protein of interest. * The average molecular weight of an amino acid is 110 daltons For ex., 1500 nucleotides means 500 codons and therefore 500 amino acids 500 x 110 = 55,000 daltons (or 55 kilodaltons, kDa). Divide this number by three to obtain the number of codons (which is the number of amino acids coded for) multiply this by 110* to obtain the approximate MW (in daltons) of your fusion protein. Begin “word count” with the first grey-shaded nucleotide, and count the entire area through and including the red “stop” TAA codon at the end of the ‘green’ GFP region.
#Procedure of western blot plus
This value can be determined by counting the nucleotides in the ‘grey’ plus ‘green’ regions of your color-coded gene diagram. In today’s procedure, you will probe your blots with antibody to the GFP ‘tag’ on your fusion proteins this will ultimately reveal a protein band whose molecular weight (MW) is a combination of the GFP protein’s MW (~26 kDa) plus that of the protein product of your gene. Western blotting (immunoblotting) to probe for GFP fusion proteins in transfected cell lysates


 0 kommentar(er)
0 kommentar(er)
